There is “flat” water and “delicious water”. There is also “healthy” water” and “less healthy” water, having nothing to do with any contaminants, byproducts of chlorine disinfection or any of that. Let’s look at these one at a time.
WHAT IS pH and HOW DOES WATER pH AFFECT HUMAN HEALTH ?
WATER and pH
Water needs no definition but what is pH?
pH , the “power of Hydrogen”, can be described by using the H2O water molecule itself. Think of the water molecule, H2O as HOH. Both H2O and HOH have two atoms of hydrogen and one oxygen atom. Let’s look at the HOH definition of the water molecule. Take the HOH apart into H + OH. In water the H atom by itself is called an ion. The OH (properly written, the [OH]- ion), by itself is another ion. The H ion (properly written, the [H]+ ion), the hydrogen ion that defines the pH.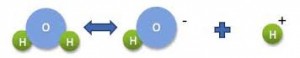
pH is the concentration of these Hydrogen ions in a water solution. Optimal Human Health needs a LOW Hydrogen Ion concentration (therefore more alkaline water) which turns out to be a HIGHER pH value.
The more Hydrogen ions in water the more acidic the water, the fewer ions, the more basic or alkaline the water becomes. The definition of pH as the negative logarithm of Hydrogen ion concentration is unnecessary here; just remember, “higher pH number, means fewer H+ ions in the water and therefore the less acidic and the more basic the water becomes”.
The pH of 7 is right in the middle. Water with pH = 7 is neutral. Optimal Alkalinity and therefore Optimal Health for human organs is between 6.5 and 8.5. More studies are emerging showing that optimal health for cellular respiration requires a more alkaline water environment for human cells, about a pH of 8.
WHAT ARE THESE DISSOLVED SOLIDS WE HEAR ABOUT? ARE THEY IMPORTANT?
Calcium (Ca++) intake is important at all ages, but the need is higher during childhood, fetal growth, pregnancy, and lactation. Epidemiological, animal, and clinical studies show that the occurrence of osteoporosis decreases as the dietary calcium intake increases. A diet that is fortified in calcium may reduce the rate of age-related bone loss and hip fractures, especially among adult women.
In spite of this knowledge, nutritional surveys indicate that more than half of North Americans consume inadequate levels of calcium and, on average, adult women consume only 60% of the required daily calcium intake. Many foods, such as orange juice, are now fortified with calcium, but naturally bioavailable calcium is found almost exclusively in milk, milk products, and water. Drinking water may be a significant source of calcium, and calcium-rich mineral water may provide over one-third of the recommended dietary intake of this mineral in adults.
Epidemiological studies also suggest that increased dietary intake of magnesium (Mg++) reduces the occurrence of schemic heart disease, cardiac arrhythmias, and sudden death. Increased levels of magnesium in drinking water are associated with decreased occurrence of cardiac disease. The majority of the U.S. population consumes less than the daily magnesium requirement, and many individuals ingest less than 80% of the recommended level. Magnesium is found in foods such as nuts, green leafy vegetables, cereals, and seafood. However, magnesium in water is highly bioavailable, and is absorbed approximately 30% faster and better than magnesium from food.[1] digm. To remain in calcium balance, net absorbed calcium (the difference between dietary intake and fecal output) has to equal calcium losses in the urine and through the skin. If that is not achieved, the calcium balance becomes negative and the difference between intake and output is drawn from the skeleton to maintain the (ionized) calcium in the extracellular fluid. Sooner or later, probably in a matter of days, this requires bone breakdown and the development of osteoporosis.[5] 150 – 300 mg / liter (ppm) concentration is the general ideal concentration of TDS (total dissolved solids) making water “taste good”
WHAT MAKES WATER “TASTY”? and is that a pH AFFECT?
Most people will say that water “tastes great” if the water’s total solids (TDS) – those things we were just discussing above – is somewhere between 200 and 350 mg/L (milligram of solid per liter of solute or water). You know of some waters you particularly like. compare them here. Look at Table 3 and Table 4 in this NIH Study done on bottled water TDS. They show the mineral content of European and North American Bottled Waters.
Find the water that you like? Now, if it is in the tables, add the numbers you see in the columns shown. Each column is the concentration of Ca++ ion, Mg++ ion and Na+ ion. the chances are good that the addition is a number between 200 and 350. After 350 mg/L, most people say the water is “too minerally” or “too hard”. IF the sum of the three minerals is less than 150 mg/L most people will say the water “tastes flat”.
What is the pH in the best tasting waters? It is usually alkaline and about 7.5 – 9 on the pH scale.
And there you have it for now.
A CAUTIONARY TALE
Nanograms or parts per trillion of contaminants in water are being shown to trigger asthma in children and arteriosclerosis in older adults. But these studies are very new and are very long baseline studies; they have taken decades to complete. In the same way, the water alkalinity for optimal human health is changing and research is pointing toward a higher pH to flush the water solvent through the cells of the liver, pancreas, kidneys, heart and brain. Certainly higher than 7.5 and perhaps as high as 9. Only more time, more studies will tell the tale.
The Surgeon General in 1964 and again in 2006 placed warning labels on cigarettes. By them he did not mean one would die tomorrow. It turns out that “dangerous to your health” meant a shorter lifespan. And that, of course, takes,….. a lifetime in order to find out, “why yes,.. that warning was right!”.

